R&D: Materials
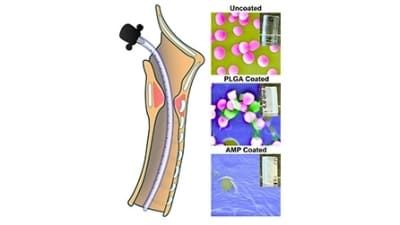
A coating can be applied to endotracheal tubes and release antimicrobial peptides that target infectious bacteria with specificity.
Features: Robotics, Automation & Control
MD&M West brings together medtech engineers, business leaders, and innovative thinkers to create life-changing medical devices.
R&D: Materials
Researchers have engineered new antimicrobial surfaces that can significantly reduce the formation of bacteria on medical instruments, such as urinary catheters, and reduce the risk of patient infection while in hospital.
R&D: Medical
An innovative system may provide a new option to use directed energy for biomedical applications.
Supplements: Motion Control
Our 2021 Resource Guide shows you the top manufacturers in materials, manufacturing, and a range of other medical-device categories.
Briefs: Medical
Next-generation sutures can deliver drugs, prevent infections, and monitor wounds.
R&D: Materials
The coatings prevent inflammation or implant rejection.
R&D: Medical
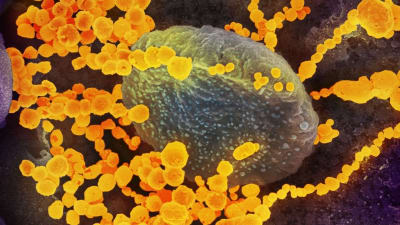
Researchers have demonstrated that a family of self-sterilizing polymers are effective at inactivating coronaviruses, including SARS-CoV-2 — the virus that causes COVID-19.
Features: Photonics/Optics
In our 2020 Product Buyer's Guide, you'll find a list of manufacturers in more than a dozen medical-device areas.
News: Packaging & Sterilization
To find out more about Cobalt-60, MDB recently spoke with Richard Wiens, who leads a team dedicated to maintaining a reliable supply of Cobalt-60 for the long term. His team manages existing supply relationships and builds new ones and currently has more than a dozen projects under way.
Products: Mechanical & Fluid Systems
Shielding materials, static control, 3D printing software, and more.
Blog: Packaging & Sterilization
NASA’s Glenn Research Center and University Hospitals (UH) in Cleveland have collaborated to develop new methods and technologies for decontaminating personal protective equipment...
Technology Leaders: Wearables
For wearable devices to function as intended, they need to have a secure, reliable hold for the desired duration of use.
Blog: Medical
FDA has created a new pilot program for industry to expedite approvals of certain changes to ethylene oxide (EtO) sterilization processes and facilities. In an effort to expedite approvals of certain changes to EtO sterilization processes and facilities, FDA is asking for...
Briefs: Packaging & Sterilization
Ultraviolet light has been used for more than 30 years as a source of disinfectant. A newly introduced product uses the technology to eliminate bacteria on cell phones, tablets and other...
Features: Medical
Updates to ISO 11607, Parts 1 and 2, have left many medical device manufacturers wondering about the future of their packaging designs. These changes come at a stressful...
News: Packaging & Sterilization
Porex, a developer of porous polymer solutions, and Guangdong Xianfeng Medical Technology Co., Ltd. have teamed up to develop a sterile container that will...
Features: Materials
Whether used in wound care products, ostomy applications, or for adhering wearable devices to skin, advanced pressure-sensitive adhesives are being created to improve breathability,...
Technology Leaders: Materials
It can be challenging to make sure you've covered all the bases during the tubing and hose selection process for medical instrumentation. For each application, there are many elements to consider, including...
News: Test & Measurement
To find out more about the expertise required to establish safe processes for cleaning and disinfecting reusable medical devices, MDB recently spoke with Christopher Scott, Vice President of Eurofins Medical Device Testing (Lancaster, PA).
Features: Design
Device functionality is usually the starting point when designing devices. Another element that needs to be considered when designing devices and their subsequent components is...
Features: Materials
Medical devices across the United States must not only keep pace with advances in technology but also with the increased use of harsh solvents and disinfectants....
Features: RF & Microwave Electronics
Ensuring patient safety and quality of care has become an increasingly technology-reliant process for most healthcare providers. With 4,000 reported “retained surgery items” cases per...
Features: Medical
When validating the steam sterilization instructions for a reusable medical device,...
Features: Materials
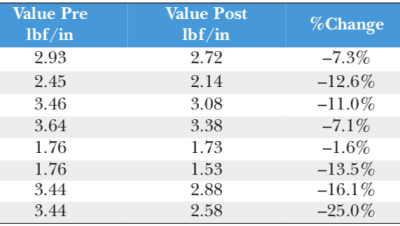
The performance of adhesives used for wearable medical device applications is critical to the efficacy of the final product, as an improperly affixed device is...
From the Editor: Motion Control
In December, we asked Medical Design Briefs readers to cast their ballot to choose from our 12 Products of the Month the technology they felt was the most significant new introduction to the design...
Technology Leaders: Packaging & Sterilization
After years of research and development, surveys and polls, functional device testing, and countless other preparatory actions, your medical device is almost ready to go to market. You have determined your...
News: Test & Measurement
Sterigenics International has changed its parent company name to Sotera Health. Its three operating companies — Nelson Labs™, Nordion®, and Sterigenics® — will continue to maintain their current names. The new parent company name signals a more integrated and holistic customer value...
News: Medical
Noxilizer, Inc. (Baltimore, MD) and Sterigenics International LLC (Broadview Heights, OH) have signed a global agreement that will make Sterigenics the exclusive worldwide provider of nitrogen dioxide (NO2) contract sterilization services, as well as feasibility and research studies,...

 Podcasts: Medical
Podcasts: Medical Podcasts: Design
Podcasts: Design Podcasts: Design
Podcasts: Design On-Demand Webinars: Medical
On-Demand Webinars: Medical Podcasts: AR/AI
Podcasts: AR/AI Podcasts: Manufacturing & Prototyping
Podcasts: Manufacturing & Prototyping