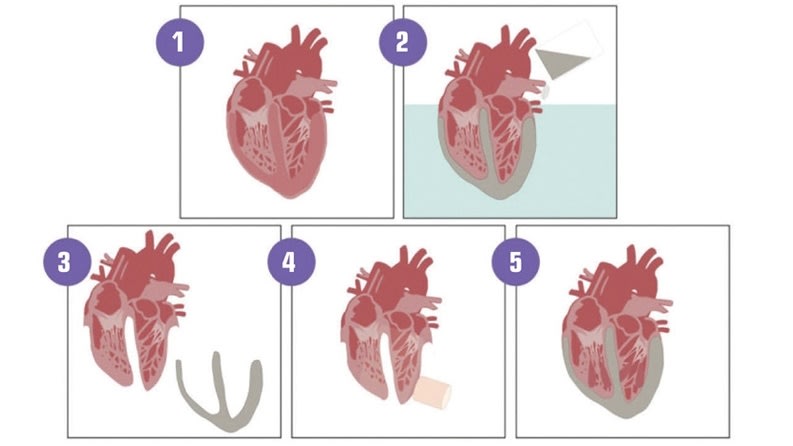
The human heart is more than a symbol of life, power, and reliability—it is a wonder of engineering achievement: a dual pump able to feed two different systems (pulmonary and cardiovascular) simultaneously, while automatically adapting itself to the activity of the body or its evolution throughout a patient’s life. It is an amazingly robust organ able to beat no fewer than 4 billion times in an average lifecycle. Yet, this masterpiece of engineering can fail: an overly calcified valve could malfunction, the left ventricle may fail to pump the blood through the entire body, or the whole heart might become unable to fulfill its vital function. (See Figure 1)
While existing artificial hearts have been in use for a few years, current FDA-approved devices are incredibly complex and large and can only be used by 20 percent of women and 50 percent of men. This technology is still considered an interim solution for those waiting for a donor. Unfortunately, 20 percent of the 300,000 Americans who die from heart failure died while waiting for a donor heart.
An Impossible Challenge to Tackle
The heart is one of the most complex organs in the human body and to engineer a working replication that can be implanted successfully for more patients takes immense creativity, as well as careful design and testing. To do so, we need to understand the key challenges to designing a reliable and comfortable solution.
Extreme Reliability: A human heart beats between 40 and 50 million times a year, which translates into a billion beats for a short (for a heart) 20-year lifespan. Herein lies one of the most critical and important challenges with artificial hearts—it must be designed with zero potential for failure for at least a billion cycles, as any failure can lead to immediate death.
Minimize Energy: An artificial heart’s dual pump needs enough energy to work properly. The natural heart is able to extract from the blood the necessary energy that is initially garnered from the food that is consumed. Although there are some thoughts that this can be replicated with electrochemistry, others are researching ways to capture renewable mechanical energy directly from the body. For example, as the patient is breathing or walking, a lot of mechanical energy from these movements can be harvested, similar to how energy is extracted from wind or the ocean tide. Current artificial hearts rely on a wirelessly rechargeable battery, which depends on the heart efficiency.
Adaptable to Activities: Even without considering the evolution of the heart as the patient ages, an artificial heart pump should adjust the blood flow based on the patient’s activities. This includes sleeping, quiet activities, or exercising, which requires sensors to properly inform the heart. Future designs must include smarter sensors to adjust necessarily to the way the heart performs and reacts to emotions, such as fear, love, or anger, and regular physical activities.
Size Matters: If all the above mentioned features are achievable, the resulting design of the artificial heart is typically so large that it cannot be implanted in an average man or woman, young adult, or child. New designs must be significantly more compact—small enough to function within a teenager’s and child’s thoracic cavity without compromising organ reliability.
Weight is More than Comfort: Power and robust performance have typically been synonymous with heavier equipment. An average adult heart weighs 300 grams while the most recent implantable artificial heart weighs nearly 900 grams. That is a 200 percent increase in weight. New engineered materials, especially composite materials, are now available that can progressively reduce the weight of the artificial hearts to closer to 300 grams. In addition, these advancements will also lead to a heart weight that is appropriate for infants.
Safety and Regulation: The heart alone is not the only consideration when designing an artificial heart. How the heart works within the body is also critical. The long-term biocompatibility of the artificial heart with surrounding soft tissues and blood are carefully scrutinized by medical regulation authorities, such as the FDA, before authorizing the implant of new equipment.
Time Pressure: Every day more than 300 people diagnosed with major heart deterioration die while waiting for a donor heart. With this in mind, an artificial heart that meets all the above criteria has the capability to save more than 100,000 patients every year.
Despite the amplitude of these challenges, medical innovators are convinced that today’s advanced technologies, such as simulation, composite materials, and miniaturization, will lead to a reliable and more broadly usable artificial heart in the near future.
Experience and Best Practices Are Keys to Success
The systematic adoption of engineering simulation by medical device companies during the past few years has dramatically accelerated the innovation process, not just for improving the quality of the device but also to make the implantation process safer. To succeed faster, companies have often adopted a few important best practices such as:
- Systematic use of engineering simulation early to accelerate the product development process and widen the optimization windows.
- Developing a computer-based comprehensive solution set as a reliable Virtual Human Laboratory to evaluate the proper behavior of the implanted device in its working environment, which usually involves soft tissues and specific pathologies.
- Considering every necessary physics in the model as it can be extremely misleading to overly simplify a medical situation. As an example, the human heart is a complex system with advanced electrical, fluid, and structure dynamics.
- Validating new virtual prototypes in the early phases of the product development process through in silico testing, such as patient specific computer-based models.
- Challenging successful new prototypes in terms of reliability and human variability while optimizing the device performance in terms of size and weight.
- Streamlining the regulatory submission process through a Simulation Assisted FDA Approval as encouraged by the Medical Device Innovation Consortium.
Combining their ingenuity with these few good practices principles, entrepreneurs have turned some concepts into real and sometimes already implanted prototypes.
Case Study: Not a Concept Anymore, But Prototypes Working in “Real Life”

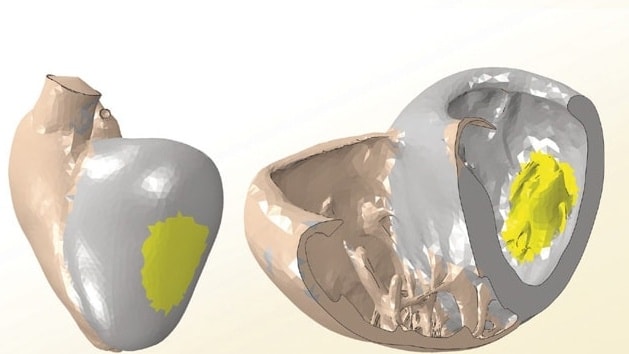
Engineering consultants from the SimuTech Group, experts in multiphysics modeling, provided guidance for the design of a new continuous-flow total artificial heart (CFTAH) that is smaller and less complex than current artificial heart devices. The goal for design simplification and increased reliability originated from a concept that would limit the number of moving parts to a single rotor suspended by a combination of magnetic and fluid forces. Limiting to a single rotor design reduces the required energy and, thus, reduces the risk of abrasion within the thoracic cavity. (See Figure 2)
During the design process, SimuTech performed multiphysics simulation to minimize the risk and number of expensive design changes of prototypes. This process included electromagnetic simulation coupled with fluid flow to fully explore the CFTAH’s operating window. As extreme reliability is one of the ultimate goals, designers needed to make sure the device works properly in every circumstance and every working condition of the CFTAH. Excluding any scenarios will increase the risk of failure, but physically testing every single scenario is not only time consuming but requires significant financial investment.
To date, simulation has been used to calculate the pump’s hydraulic performance, static pressures, rotor torque, and other key parameters, all as part of the process of ensuring product design robustness before animal testing. Simulation is also needed to capture data that cannot be collected during physical testing or to evaluate design alternatives in less time and at a lower cost than could be accomplished with physical testing.
The CFTAH’s design (during normal operation) allows the rotor to freely move axially. Its axial position is determined by the magnet’s axial restoring force and opposite-acting left and right side pump pressures. When the right pump pressure is higher than the left pump pressure, the rotating assembly is shifted to the left by hydraulic forces. This leftward shift increases the size of the right pump aperture, which increases the right pump’s output pressure and flow rate. The increase in right pump performance raises the pressure and flow rate entering the left pump, which increases the left pump pressure and causes the rotating assembly to shift back rightwards. This self-regulation process automatically corrects any imbalances between the right and left side pumps.
Multiphysics simulation was required because of the importance of both electromagnetic forces and fluid flow in determining pump performance. A 3D electromagnetic model was developed with simulation software from ANSYS, Inc., to predict the magnetic radial and axial forces and torques acting on the rotor while a 3D fluid mechanical model of the heart used the calculated magnetic forces to identify the rotor radial position at various rotor speeds.
Electromagnetics simulation predicted the radial and axial forces generated by the magnet that moves the rotor toward the centerline position as a function of the offset from the center. The design depends on restoring forces to help control and limit the position of the rotor. The simulation showed that the magnet produces the necessary restoring force to compensate for offset from the centerline of the bearing.
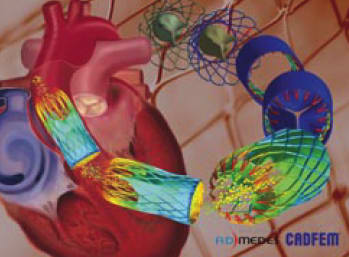
Before considering in vivo testing, it was essential to gain confidence by comparing in vitro testing with in silico investigations. The research team modeled the complete CFTAH pump assembly and compared its performance to physical test results using the same water/glycerin mixture as the fluid. The model was evaluated at three different volumetric flow rates and three different rotational speeds spanning the intended range of use. Multiphysics simulations predicted hydraulic performance, surface static pressures, and rotor torque within 5 to 10 percent of the prototype’s measurements. The axial position of the rotating assembly predicted by simulation matched experimental measurements within 0.25 mm. (See Figure 3)

In a more sophisticated simulation model under development, the team will use blood as the fluid, enabling examination of the shear forces exerted on the blood by the pump’s surfaces to get closer to the in vivo model. Shear forces need to be closely controlled. If they are too high, the blood cells can be damaged and if too low, the blood can clot.
The next few steps in this process include pre-clinical testing followed by clinical testing. The hope is that these results will instill great confidence in the artificial heart’s potential performance before formal approval by the FDA and eventually the release of this vital innovation to the market within a few years.
What Should We Expect?
If we compare artificial hearts to the evolution of computers, cell phones, or pacemakers, we have no doubt that artificial hearts will soon address and overcome the challenges described above. With the use of key technologies such as simulation, artificial hearts will be more compact and lighter. In addition, these engineered hearts will become “smarter” with the ability to adjust blood flow to the needs of its surrounding environment (the body and beyond). Smarter could also suggest being able to extract energy from the body and use it, identifying potential troubles to call for a light maintenance, and preventing the emergence of life-threatening problems. With continued investment, development, and globalization of artificial hearts, the associated cost is also expected to decrease dramatically, making it more affordable across the world. All these the lessons learned from the design of artificial hearts is also spreading to other organizations focused on designing artificial lungs, livers, kidneys, and other essential organs.
More importantly, they will be able to meet broader, patient-specific requirements from adults, to adolescents, to newborns.
This article was written by Thierry Marchal, Industry Director for Healthcare at ANSYS, Inc., Canonsburg, PA. For more information, Click Here " target="_blank" rel="noopener noreferrer">http://info.hotims.com/49744-164.